
Chemistry, 15.11.2019 00:31 siicklmods4295
The generic metal hydroxide m(oh)2 has ksp = 7.65×10−12. (note: in this particular problem, because of the magnitude of the ksp and the stoichiometry of the compound, the contribution of oh− from water can be ignored. however, this may not always be the case.) part a what is the solubility of m(oh)2 in pure water? express your answer with the appropriate units

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
The generic metal hydroxide m(oh)2 has ksp = 7.65×10−12. (note: in this particular problem, because...
Questions

Computers and Technology, 23.09.2020 17:01



Mathematics, 23.09.2020 17:01





Spanish, 23.09.2020 17:01

Computers and Technology, 23.09.2020 17:01




Computers and Technology, 23.09.2020 17:01



Social Studies, 23.09.2020 17:01


Social Studies, 23.09.2020 17:01

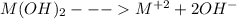
![[M^{+2}][OH^{-}]^{2}](/tpl/images/0374/9060/f9163.png)



