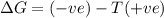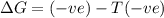Chemistry, 15.11.2019 02:31 williejaroid123
Classify the possible combinations of signs for a reaction's δh and δs values by the resulting spontaneity
a. δh is positive and δs is negative
b. δh is positive and δs is positive
c. δh is negative and δs is positive
d. δh negative and δs is negative
for a, b, c and d find out which of following they are:
1. spontaneous as written at all temperatures
2. spontaneous in reverse at all temperatures
3. spontaneous as written above a certain temperature
4. spontaneous as written below a certain temperature
they can only have one answer but 2 can have the same answer

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Classify the possible combinations of signs for a reaction's δh and δs values by the resulting spont...
Questions


Health, 17.09.2019 21:00

Mathematics, 17.09.2019 21:00

Mathematics, 17.09.2019 21:00

Mathematics, 17.09.2019 21:00

History, 17.09.2019 21:00

History, 17.09.2019 21:00


Mathematics, 17.09.2019 21:00


Mathematics, 17.09.2019 21:00


History, 17.09.2019 21:00

History, 17.09.2019 21:00


Geography, 17.09.2019 21:00




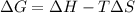
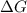 = Gibbs free energy
= Gibbs free energy 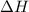 = enthalpy change
= enthalpy change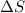 = entropy change
= entropy change 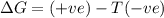
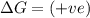
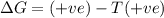
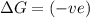 (at high temperature) (spontaneous)
(at high temperature) (spontaneous)