
Chemistry, 15.11.2019 04:31 hannahgracew12
Consider the equilibrium reaction below. at high concentrations of ha the solution will be yellow; but at low concentrations of ha the solution will be red. initially the equilibrium mixture below is orange (combination of yellow & red). what color is expected after adding some colorless a–(aq) to the equilibrium solution? a-(aq) + h2o(ℓ) ⇋ ha(aq) + oh-(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Consider the equilibrium reaction below. at high concentrations of ha the solution will be yellow;...
Questions


History, 04.07.2019 11:30



Mathematics, 04.07.2019 11:30








Mathematics, 04.07.2019 11:30





Biology, 04.07.2019 11:30


Mathematics, 04.07.2019 11:30

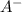 is added into the solution then it means that we are increasing the amount of reactant.
is added into the solution then it means that we are increasing the amount of reactant. 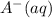 to the equilibrium solution.
to the equilibrium solution.

