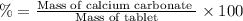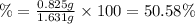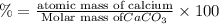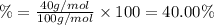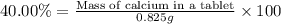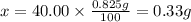Chemistry, 15.11.2019 07:31 harmonyfern5648
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. after the fillers were filtered out, the hcl was neutralized by adding sodium carbonate. the resulting precipitate was pure calcium carbnonate (with the fillers removed). the solid calcium carbonate was collected on a watch glass that had a mass of 46.719 g when empty. after teh calcium carbonate had been allowed to dry, the mass of the watch glass plus product was found to be 47.544 g.
1. what is the mass of pure calcium carbonate product collected at the end of the experiment?
2. calculate the mass % calium carbonate in the tablet.
3. calculate the mass percent calcium in calcium carbonate. this calculation is theoretical and is independent of the data provided.
4. calculate the number of grams of calcium that were in the tablet.
(hint: this can be obtained by using the answers to question 1 and 3)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. afte...
Questions



Mathematics, 09.10.2021 20:10

History, 09.10.2021 20:10

English, 09.10.2021 20:10


English, 09.10.2021 20:10

SAT, 09.10.2021 20:10



Chemistry, 09.10.2021 20:10


Mathematics, 09.10.2021 20:10




Mathematics, 09.10.2021 20:10

Mathematics, 09.10.2021 20:10