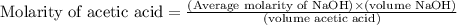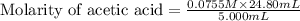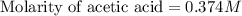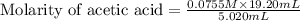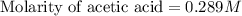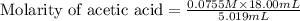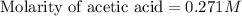Chemistry, 15.11.2019 18:31 beckers0115
Calculate and enter the molarity of your three acetic acid trials using the volume of standardized naoh solution required for each and the average molarity of the naoh solution from the standardization trials with khp. you should report 3 significant figures, e. g. 0.488 m.
entry # vol acetic acid(ml) vol na0h(ml) m acetic acid
#1: 5.000 24.80
#2: 5.020 19.20
#3: 5.019 18.00

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

You know the right answer?
Calculate and enter the molarity of your three acetic acid trials using the volume of standardized n...
Questions

Mathematics, 23.10.2020 14:40


Mathematics, 23.10.2020 14:40





Mathematics, 23.10.2020 14:40

Mathematics, 23.10.2020 14:40




Social Studies, 23.10.2020 14:40

Mathematics, 23.10.2020 14:40

English, 23.10.2020 14:40

Mathematics, 23.10.2020 14:40

Mathematics, 23.10.2020 14:40

History, 23.10.2020 14:40