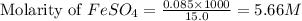In an oxidation-reduction reaction, it required 25.6 ml of a 0.65 m potassium permanganate solution to reach the equivalence point with 15.0 ml of an iron(ii) sulfate solution. what is the molar concentration of the iron(ii) sulfate solution? the net ionic equation for the reaction is:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
In an oxidation-reduction reaction, it required 25.6 ml of a 0.65 m potassium permanganate solution...
Questions

History, 21.09.2019 16:10



History, 21.09.2019 16:10





Physics, 21.09.2019 16:10



Biology, 21.09.2019 16:10


Mathematics, 21.09.2019 16:10

History, 21.09.2019 16:10

Mathematics, 21.09.2019 16:10




 .....(1)
.....(1)

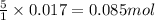 of iron (II) ions
of iron (II) ions