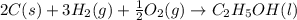Chemistry, 15.11.2019 20:31 johnnybones03
The enthalpy of formation of liquid ethanol (c2h5oh) is −277.6 kj/mol. what is the equation that represents the formation of liquid ethanol? a. 2 c(s) + 6 h(g) + o(g) → c2h5oh(l) b. 2 c(s) + 3 h2(g) + ½ o2(g) → c2h5oh(l) c. 2co2(g) + 3h2o(g) → c2h5oh(l) + 3 o2(g) d. 4 c(s) + 6 h2(g) + o2(g) → 2 c2h5oh(l)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Which of these reactions are redox reactions? check all that apply.cd + hcl → cdcl2 + h2cucl2 + na2s → 2nacl + cuscaco3 → cao + co2 2zns + 3o2 → 2zno + 2so2 ch4 + 2o2 → co2 + 2h2o
Answers: 3

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
The enthalpy of formation of liquid ethanol (c2h5oh) is −277.6 kj/mol. what is the equation that rep...
Questions

Chemistry, 08.10.2021 14:00

World Languages, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

Biology, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

French, 08.10.2021 14:00

Social Studies, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

Computers and Technology, 08.10.2021 14:00


History, 08.10.2021 14:00




Mathematics, 08.10.2021 14:00

History, 08.10.2021 14:00

History, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00