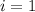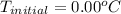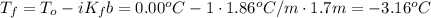Chemistry, 15.11.2019 20:31 elizabethhubbe
What would be the freezing point of a 1.7 mole aqueous ethylene glycol solution? the freezing point depression constant for water = 1.86 degrees celsius per mole.
a) 3.2°c
b) – 1.1°c
c) 0.0°c
d) – 3.2°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
What would be the freezing point of a 1.7 mole aqueous ethylene glycol solution? the freezing point...
Questions


English, 06.03.2021 14:00

History, 06.03.2021 14:00


English, 06.03.2021 14:00



Mathematics, 06.03.2021 14:00

Biology, 06.03.2021 14:00


Spanish, 06.03.2021 14:00


Biology, 06.03.2021 14:00

Mathematics, 06.03.2021 14:00

History, 06.03.2021 14:00

English, 06.03.2021 14:00



Mathematics, 06.03.2021 14:00


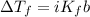
 is the change in the freezing point of the solvent given its initial and final freezing point temperature values;
is the change in the freezing point of the solvent given its initial and final freezing point temperature values; is the van 't Hoff factor (i = 1 for non-electrolyte solutes and i depends on the number of moles of ions released per mole of ionic salt);
is the van 't Hoff factor (i = 1 for non-electrolyte solutes and i depends on the number of moles of ions released per mole of ionic salt); is the freezing point depression constant for the solvent;
is the freezing point depression constant for the solvent; is molality of the solute, defined as a ratio between the moles of solute and the mass of solvent (in kilograms).
is molality of the solute, defined as a ratio between the moles of solute and the mass of solvent (in kilograms).