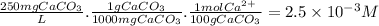The buffer solution is used to control the ph to insure that it does not become too high because excessively basic solutions could cause the corresponding hydroxides of hard metal ions (such as ca(oh)2 and mg(oh)2) to precipitate. using the calcium ion as a typical representative, just how high a ph do you think could be considered as "too high" for a solution with a hardness of about 250 ppm caco3? ksp for ca(oh)2 is 6.5 x 10-6.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:50
What is the specific heat of a substance that absorbs 2.5×10^3 joules of heat when a sample of 1.0 ×10^4g of the substance increases in temperature from 10°c to 70°c?
Answers: 2

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1
You know the right answer?
The buffer solution is used to control the ph to insure that it does not become too high because exc...
Questions






Geography, 22.10.2020 16:01



Mathematics, 22.10.2020 16:01





Mathematics, 22.10.2020 16:01




Geography, 22.10.2020 16:01

History, 22.10.2020 16:01

Chemistry, 22.10.2020 16:01