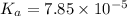Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
1.50 g of a weak acid (molar mass 176) is dissolved in 50.0 ml of water, and the resultant solution...
Questions


Mathematics, 22.03.2021 17:40






Mathematics, 22.03.2021 17:40




Mathematics, 22.03.2021 17:40



Mathematics, 22.03.2021 17:40





Mathematics, 22.03.2021 17:40

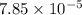 .
.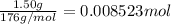
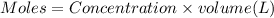
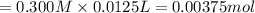
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0376/5102/e4eea.png)

![pH=pK_a+\log(\frac{[NaA]}{[HA]})](/tpl/images/0376/5102/83d67.png)
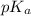 = ?
= ?![[HA]=\frac{0.008523 mol-0.00375}{0.0625 L}=\frac{0.004773 mol}{0.0625 L}](/tpl/images/0376/5102/6fe77.png)
![[NaA]=\frac{0.00375 mol}{0.0625 L}](/tpl/images/0376/5102/93c44.png)
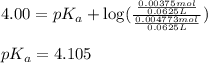
![4.105=-\log[K_a]](/tpl/images/0376/5102/b305f.png)