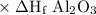Calculate the standard enthalpy of the reaction, δh∘rxn, for the thermite reaction:
2al(s)+fe2o3(s)→2fe(s)+al2o3(s)
elements in their standard state have an enthalpy of formation value of zero. the standard enthalpies of formation of fe2o3 and al2o3 are
δh∘f of fe2o3(s)=−825.5 kj/molδh∘f of al2o3(s)=−1675 kj/mol
express your answer to three significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
How many joules of heat are absorbed to raise the temperature of 650 grams of water from 5.00c to it's boiling point, 100c
Answers: 1

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Calculate the standard enthalpy of the reaction, δh∘rxn, for the thermite reaction:
2al(s)+fe...
2al(s)+fe...
Questions

Computers and Technology, 20.03.2020 22:09



Mathematics, 20.03.2020 22:10

Biology, 20.03.2020 22:10

Mathematics, 20.03.2020 22:10

Mathematics, 20.03.2020 22:10

English, 20.03.2020 22:11


Mathematics, 20.03.2020 22:11

Mathematics, 20.03.2020 22:11

Chemistry, 20.03.2020 22:11





Mathematics, 20.03.2020 22:12

Mathematics, 20.03.2020 22:12

History, 20.03.2020 22:12

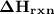 ) can be given by:
) can be given by:
 + 1
+ 1 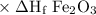
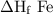 + 1
+ 1