
In methane combustion, the following reaction pair is important: at 1500 k, the equilibrium constant kp has a value of 0.003691 based on a reference-state pressure of 1 atm (101,325 pa). derive an algebraic ex- pression for the forward rate coefficient kf . evaluate your expression for a temperature of 1500 k. give units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1

Chemistry, 23.06.2019 13:00
Sort these isotopes by whether they are most likely to undergo fusion or fission. hydrogen-3, uranium-233, plutonium-239, hydrogen-1, helium-3, plutonium-241
Answers: 2
You know the right answer?
In methane combustion, the following reaction pair is important: at 1500 k, the equilibrium constan...
Questions


English, 28.10.2019 23:31







Physics, 28.10.2019 23:31




History, 28.10.2019 23:31


History, 28.10.2019 23:31

Physics, 28.10.2019 23:31



History, 28.10.2019 23:31

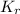 =
= 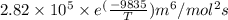
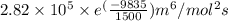
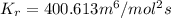
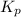 and
and 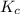 is as follows.
is as follows.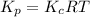
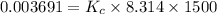
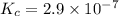
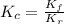
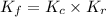
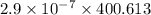
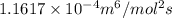
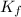 is
is 

