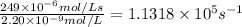If the enzyme molecule has more than one active site, then [e]t is multiplied by the number of active sites to determine its effective concentration. determine the value of the turnover number of the enzyme carbonic anhydrase, given that for carbonic anhydrase equals 249 μmol⋅l−1⋅s−1 and [e]t=2.20 nmol⋅l−1 . carbonic anhydrase has a single active site. turnover number =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
If the enzyme molecule has more than one active site, then [e]t is multiplied by the number of activ...
Questions



Physics, 28.07.2019 10:00


Biology, 28.07.2019 10:00



Mathematics, 28.07.2019 10:00


English, 28.07.2019 10:00

History, 28.07.2019 10:00




Health, 28.07.2019 10:00


History, 28.07.2019 10:00



Social Studies, 28.07.2019 10:00

 .
.![\frac{R_{max}}{[E]_t}](/tpl/images/0376/6860/df5a6.png)
![[E]_t](/tpl/images/0376/6860/793ae.png) =catalytic site concentration
=catalytic site concentration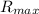 = Maximum reaction rate
= Maximum reaction rate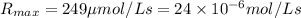
![[E]_t=2.20 nmol/L=2.20\times 10^{-9} mol/L](/tpl/images/0376/6860/17661.png)