
High-purity benzoic acid (c6h5cooh; δhrxn for combustion = −3227 kj/mol) is used as a standard for calibrating bomb calorimeters. a 1.221-g sample burns in a calorimeter (heat capacity = 1365 j/°c) that contains exactly 1.430 kg of water. what temperature change is observed?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2


Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
High-purity benzoic acid (c6h5cooh; δhrxn for combustion = −3227 kj/mol) is used as a standard for...
Questions






Computers and Technology, 14.07.2020 19:01





English, 14.07.2020 19:01





Computers and Technology, 14.07.2020 19:01

History, 14.07.2020 19:01



English, 14.07.2020 19:01

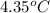
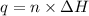
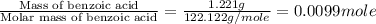
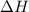 = enthalpy of combustion = 3227 kJ/mole
= enthalpy of combustion = 3227 kJ/mole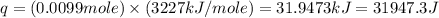
![q=[q_1+q_2]](/tpl/images/0376/8173/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0376/8173/1d50b.png)
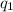 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter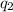 = heat absorbed by the water
= heat absorbed by the water = specific heat of calorimeter =
= specific heat of calorimeter = 
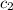 = specific heat of water =
= specific heat of water = 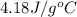
 = mass of water = 1.430 kg = 1430 g
= mass of water = 1.430 kg = 1430 g = change in temperature = ?
= change in temperature = ?![31947.3J=[(1365J/^oC\times \Delta T)+(1430g\times 4.18J/g^oC\times \Delta T)]](/tpl/images/0376/8173/c2161.png)



