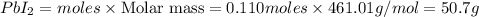Consider the balanced equation of k i ki reacting with p b ( n o 3 ) 2 pb(nox3)x2 to form a precipitate. 2 k i ( a q ) + p b ( n o 3 ) 2 ( a q ) ⟶ p b i 2 ( s ) + 2 k n o 3 ( a q ) 2ki(aq)+pb(nox3)x2(aq)⟶pbix2(s)+2kn ox3(aq) what mass of p b i 2 pbix2 can be formed by adding 0.528 l of a 0.417 m solution of k i ki to a solution of excess p b ( n o 3 ) 2 pb(nox3)x2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

You know the right answer?
Consider the balanced equation of k i ki reacting with p b ( n o 3 ) 2 pb(nox3)x2 to form a precipit...
Questions

Mathematics, 20.09.2020 18:01



History, 20.09.2020 18:01






Mathematics, 20.09.2020 18:01



Mathematics, 20.09.2020 18:01


Mathematics, 20.09.2020 18:01





English, 20.09.2020 18:01

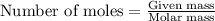
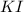
![\text{Number of moles}=molarity\times {\text {Volume in L]}=0.417M\times 0.528L=0.220moles](/tpl/images/0376/8666/592a1.png)
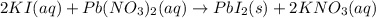
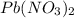 is in excess.
is in excess.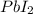
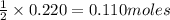 of
of