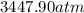Chemistry, 16.11.2019 03:31 prettyluhangel
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by the van der waals equation? the ideal gas law constant is 0.08206 [l•atm] / [mol•k]. for co2, the pressure correction constant is 3.658 l2•atm / mol 2, and the volume correction constant is 0.04286 l / mol.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by th...
Questions



Biology, 23.07.2019 20:00

Mathematics, 23.07.2019 20:00






Social Studies, 23.07.2019 20:00




Spanish, 23.07.2019 20:00


Mathematics, 23.07.2019 20:00




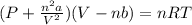
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}][1.0L-(20.0mol\times0.04286 L.mol^{-1} )]=(20.0mol)\times (0.08206L.atm.mol^{-1}.K^{-1})\times (300.0K)](/tpl/images/0376/9858/97073.png)
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}]](/tpl/images/0376/9858/353d5.png) =
=