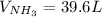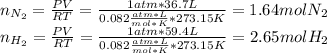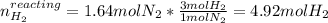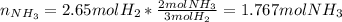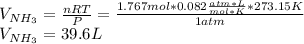Chemistry, 16.11.2019 03:31 kedjenpierrelouis
Consider the reaction between hydrogen gas and nitrogen gas to form ammonia: 3 h2(g) + n2(g) → 2 nh3(g). what volume of ammonia (in l) could be produced by the reaction of 59.4 liters of hydrogen with 36.7 liters of nitrogen at a constant pressure and temperature?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
Consider the reaction between hydrogen gas and nitrogen gas to form ammonia: 3 h2(g) + n2(g) → 2 nh...
Questions

Social Studies, 14.10.2019 01:30


Mathematics, 14.10.2019 01:30

Social Studies, 14.10.2019 01:30




Social Studies, 14.10.2019 01:30



History, 14.10.2019 01:30

History, 14.10.2019 01:30


Mathematics, 14.10.2019 01:30

Business, 14.10.2019 01:30



Biology, 14.10.2019 01:30