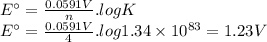The overall reaction and equilibrium constant value for a hydrogen-oxygen fuel cell at 298 k is given below. 2 h2(g) + o2(g) → 2 h2o(l) k = 1.34 ✕ 1083 (a) calculate ℰ° and δg° at 298 k for the fuel-cell reaction. ℰ° v δg° kj (b) predict the signs of δh° and δs° for the fuel-cell reaction. δh°: positive negative δs°: positive negative (c) as temperature increases, does the maximum amount of work obtained from the fuel-cell reaction increase, decrease, or remain the same? explain. since δs is , as t increases, δg becomes more . therefore, the maximum work obtained will as t increases.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
The overall reaction and equilibrium constant value for a hydrogen-oxygen fuel cell at 298 k is give...
Questions

Physics, 21.09.2019 08:20

History, 21.09.2019 08:20

Geography, 21.09.2019 08:20


Mathematics, 21.09.2019 08:20


Mathematics, 21.09.2019 08:20

History, 21.09.2019 08:20

Chemistry, 21.09.2019 08:20

English, 21.09.2019 08:20


Mathematics, 21.09.2019 08:20


Social Studies, 21.09.2019 08:20

Mathematics, 21.09.2019 08:20



Physics, 21.09.2019 08:20

History, 21.09.2019 08:20