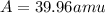Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic mass of argon to two decimal places, given the following relative atomic masses and the abundances of each of the isotopes: argon36 (35.97 amu; 0.337%), argon-38 (37.96 amu; 0.063%), argon-40 (39.96 amu; 99.600%). 1. 119.89 amu 2. 39.95 amu 3. 39.96 amu 4. 35.97 amu 5. none of these 6. 37.96 amu 7. 37.95 amu 8. 35.96 amu

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 22.06.2019 00:30
Lem 2 the data below are for the system ethyl propyl ether (1)-chloroform (2) at 0.5 bar. use the data to answer the following questions (all questions refer to p d 0: 5 bar). a) what are the boiling points of the pure components at 0.5 bar? b) a mixture with the overall composition z1 d 0: 1 is brought to 47.6ä±c, 0.5 bar. what is the phase? c) 100 mole of a mixture with z1 d 0: 1 (state a) is mixed with 22 mole of pure ethyl propyl ether vapor (state b). the mixing takes place at 47.6 ä±c, 0.5. bar. what is the phase of the resulting mixture (state c)? if the state is a v/l mixture report the number of moles and mole fractions in each phase. d) plot the txy graph and show states a, b and c. the graph must be done by computer and should be properly annotated. ethyl propyl ether (1) - chloroform (2) at 0.5 bar t ( ä±c) x1 y1 t ( ä±c) x1 y1 42.9 0.000 0.000 49.0 0.470 0.455 43.0 0.020 0.010 49.1 0.520 0.520 43.9 0.065 0.029 48.9 0.567 0.592 45.4 0.156 0.089 48.3 0.652 0.720 46.4 0.215 0.142 47.6 0.745 0.815 47.6 0.296 0.223 46.7 0.822 0.872 48.3 0.362 0.302 45.7 0.907 0.937 48.7 0.410 0.375 44.6 1.000
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic ma...
Questions

Chemistry, 07.12.2021 22:20

Chemistry, 07.12.2021 22:20


Social Studies, 07.12.2021 22:20

Mathematics, 07.12.2021 22:20


Computers and Technology, 07.12.2021 22:20

History, 07.12.2021 22:20

Mathematics, 07.12.2021 22:20


Biology, 07.12.2021 22:20

Mathematics, 07.12.2021 22:20


Mathematics, 07.12.2021 22:20

Computers and Technology, 07.12.2021 22:20




Mathematics, 07.12.2021 22:20

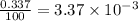
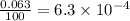
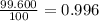
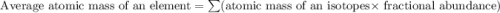
![A=\sum[(35.97\times 3.37\times 10^{-3})+(37.96\times 6.3\times 10^{-4})+(39.96\times 0.996)]](/tpl/images/0377/0178/15521.png)