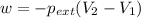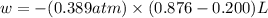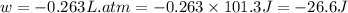Chemistry, 16.11.2019 05:31 INEEDHELP6845
Ascientist observed that a gas expanded from a volume of 0.200 l to a volume of 0.876 l. (a) what is the amount of work (in joules) performed in this process if the gas expanded at a constant pressure of 0.389 atm? w = j (b) if the temperature of the gas did not change during the expansion, calculate the change in internal energy of the gas, as well as the change in heat due to the expansion. δe = j q = j

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

You know the right answer?
Ascientist observed that a gas expanded from a volume of 0.200 l to a volume of 0.876 l. (a) what is...
Questions

Mathematics, 31.12.2019 02:31



Mathematics, 31.12.2019 02:31

Mathematics, 31.12.2019 02:31





Mathematics, 31.12.2019 02:31

Mathematics, 31.12.2019 02:31

English, 31.12.2019 02:31

Mathematics, 31.12.2019 02:31



Mathematics, 31.12.2019 02:31

Biology, 31.12.2019 02:31

Health, 31.12.2019 02:31


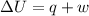
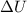 = internal energy
= internal energy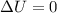
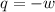
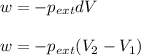
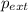 = external pressure = 0.389 atm
= external pressure = 0.389 atm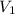 = initial volume of gas = 0.200 L
= initial volume of gas = 0.200 L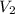 = final volume of gas = 0.876 L
= final volume of gas = 0.876 L