
Chemistry, 16.11.2019 05:31 andregijoe41
What is the molarity of zncl2 that forms when 25.0 g of zinc completely reacts with cucl2 according to the following reaction? assume a final volume of 285 ml .
zn(s)+cucl2(aq)→zncl2(aq)+cu(s)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
What is the molarity of zncl2 that forms when 25.0 g of zinc completely reacts with cucl2 according...
Questions

Mathematics, 02.12.2021 03:00


English, 02.12.2021 03:00

Computers and Technology, 02.12.2021 03:00



Mathematics, 02.12.2021 03:00




Mathematics, 02.12.2021 03:00


Biology, 02.12.2021 03:00

Computers and Technology, 02.12.2021 03:00

Computers and Technology, 02.12.2021 03:00

English, 02.12.2021 03:00




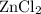 is 1.364 M
is 1.364 M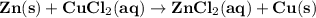
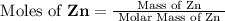 , molar mass of Zn = 65.38 g/mol
, molar mass of Zn = 65.38 g/mol 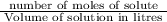
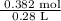 = 1.364 M.
= 1.364 M.

