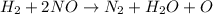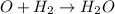Chemistry, 16.11.2019 06:31 arodriguez395
2h2(g) + 2no(g) → n2(g) + 2h2o(g) is rate=k[h2][no]2 (at least at low concentrations of h2). a possible mechanism for this reaction with two steps and an oxygen atom intermediate might take the form: h2 + 2no → n2 + x + o (step 1; k1) o + y → z (step 2; k2)
a. identify the chemical species x, y, and z in the mechanism.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
2h2(g) + 2no(g) → n2(g) + 2h2o(g) is rate=k[h2][no]2 (at least at low concentrations of h2). a possi...
Questions




Social Studies, 04.05.2021 22:00



Mathematics, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00




English, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00






Mathematics, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00

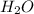 ,
, 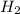 respectively.
respectively.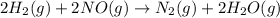
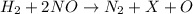
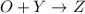
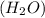 on right side of the reaction. So, the reaction 1 will be:
on right side of the reaction. So, the reaction 1 will be: