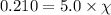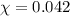Chemistry, 16.11.2019 07:31 Tyasiapalair371
Assume a scuba diver is working at a depth where the total pressure is 5.0 atm (about 50 m below the surface). what mole fraction of oxygen is necessary in order for the partial pressure of oxygen in the gas mixture the diver breathes to be 0.210 atm?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Assume a scuba diver is working at a depth where the total pressure is 5.0 atm (about 50 m below the...
Questions



Mathematics, 30.08.2019 03:00

Social Studies, 30.08.2019 03:00





Mathematics, 30.08.2019 03:00

Geography, 30.08.2019 03:00


Social Studies, 30.08.2019 03:00

Mathematics, 30.08.2019 03:00



History, 30.08.2019 03:00


Mathematics, 30.08.2019 03:00


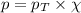
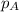 = partial pressure of oxygen = 0.210 atm
= partial pressure of oxygen = 0.210 atm = total pressure = 5.0 atm
= total pressure = 5.0 atm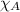 = mole fraction of oxygen = ?
= mole fraction of oxygen = ?