

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3


Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
What would be the freezing point of a 1.7 mole aqueous ethylene glycol solution? the freezing point...
Questions




History, 07.05.2020 05:12

Social Studies, 07.05.2020 05:12

Geography, 07.05.2020 05:12



Computers and Technology, 07.05.2020 05:12



Mathematics, 07.05.2020 05:12





Mathematics, 07.05.2020 05:12



English, 07.05.2020 05:12

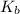 = 1.8 deg C/m
= 1.8 deg C/m
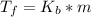
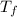 =1.8* 1.7
=1.8* 1.7


