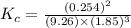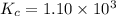Amixture of hydrogen and nitrogen, which produces ammonia (nh3) in a reaction vessel, is allowed to reach equilibrium at a given temperature. the equilibrium mixture of gases contained 0.254 m nh3, 1.85 m n2, and 9.26 m h2. calculate the equilibrium constant, kc at this temperature.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
Amixture of hydrogen and nitrogen, which produces ammonia (nh3) in a reaction vessel, is allowed to...
Questions


History, 22.11.2019 23:31



Mathematics, 22.11.2019 23:31



Social Studies, 22.11.2019 23:31



Physics, 22.11.2019 23:31



History, 22.11.2019 23:31


History, 22.11.2019 23:31

History, 22.11.2019 23:31



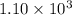
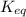
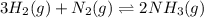
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0379/6771/c3aa0.png)