
Chemistry, 18.11.2019 18:31 hallkanay7398
6) at elevated temperatures, methylisonitrile (ch3nc) isomerizes to acetonitrile (ch3cn): ch3nc (g) ch3cn (g)at the start of an experiment, there are 0.200 mol of reactant and 0 mol of product in the reaction vessel. after 25 min, 0.108 mol of reactant (ch3nc) remain. there are mol of product (ch3cn) in thereaction vessel. a) 0.200 b) 0.540 c) 0.022 d) 0.092 e) 0.308

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
6) at elevated temperatures, methylisonitrile (ch3nc) isomerizes to acetonitrile (ch3cn): ch3nc (g)...
Questions





History, 25.07.2019 14:30

English, 25.07.2019 14:30

History, 25.07.2019 14:30




Mathematics, 25.07.2019 14:30


Health, 25.07.2019 14:30



English, 25.07.2019 14:30

Mathematics, 25.07.2019 14:30


Mathematics, 25.07.2019 14:30

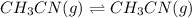
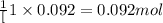 of product
of product

