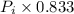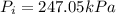Chemistry, 18.11.2019 19:31 hadilalhjajih
An apparatus consists of a 4 l flask containing nitrogen gas at 27◦c and 875 kpa, joined by a valve to a 13 l flask containing argon gas at 27◦c and 54.9 kpa. the valve is opened and the gases mix. what is the partial pressure of nitrogen after mixing? answer in units of kpa.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Forests and meadows are often cut down to make way for farms or large number of new homes. what are some of the elements of ecosystems that are lost when plants in these areas are removed?
Answers: 2

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
An apparatus consists of a 4 l flask containing nitrogen gas at 27◦c and 875 kpa, joined by a valve...
Questions

Social Studies, 02.10.2019 06:30

English, 02.10.2019 06:30






Mathematics, 02.10.2019 06:30

Geography, 02.10.2019 06:30





Mathematics, 02.10.2019 06:30



Mathematics, 02.10.2019 06:30

Biology, 02.10.2019 06:30


Social Studies, 02.10.2019 06:30

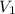 = 4 L,
= 4 L, 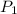 = 875 kPa
= 875 kPa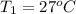 = (27 + 273) K = 300 K,
= (27 + 273) K = 300 K, 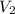 = 13 L
= 13 L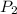 = 54.9 kPa,
= 54.9 kPa, 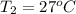
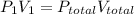 (as T is same so it will cancel out)
(as T is same so it will cancel out)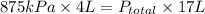
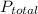 = 205.8 kPa
= 205.8 kPa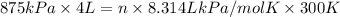
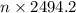
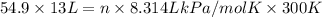
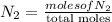
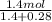
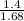
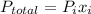
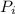 = partial pressure of a gas
= partial pressure of a gas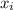 = mole fraction of the gas
= mole fraction of the gas