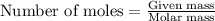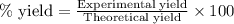Chemistry, 18.11.2019 19:31 puchie1225
A15.0 ml sample of a 1.92 m potassium sulfate solution is mixed with 14.9 ml of a 0.860 m barium nitrate solution and this precipitation reaction occurs: k2so4(aq) ba(no3)2(aq)→baso4(s) 2kno3(aq) the solid baso4 is collected, dried, and found to have a mass of 2.46 g . determine the limiting reactant, the theoretical yield, and the percent yield. part a determine the limiting reactant. express your answer as a chemical formula.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
A15.0 ml sample of a 1.92 m potassium sulfate solution is mixed with 14.9 ml of a 0.860 m barium nit...
Questions

Engineering, 25.01.2021 01:30

Mathematics, 25.01.2021 01:30

Mathematics, 25.01.2021 01:30



Mathematics, 25.01.2021 01:30

Mathematics, 25.01.2021 01:30

Mathematics, 25.01.2021 01:30






Biology, 25.01.2021 01:30

Mathematics, 25.01.2021 01:30

Mathematics, 25.01.2021 01:30

Mathematics, 25.01.2021 01:30

Mathematics, 25.01.2021 01:30


History, 25.01.2021 01:30

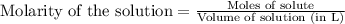 .....(1)
.....(1)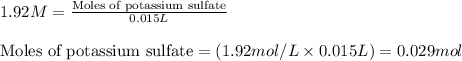

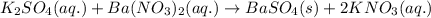
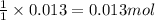 of potassium sulfate.
of potassium sulfate.