Chemistry, 18.11.2019 19:31 brittanyfox411
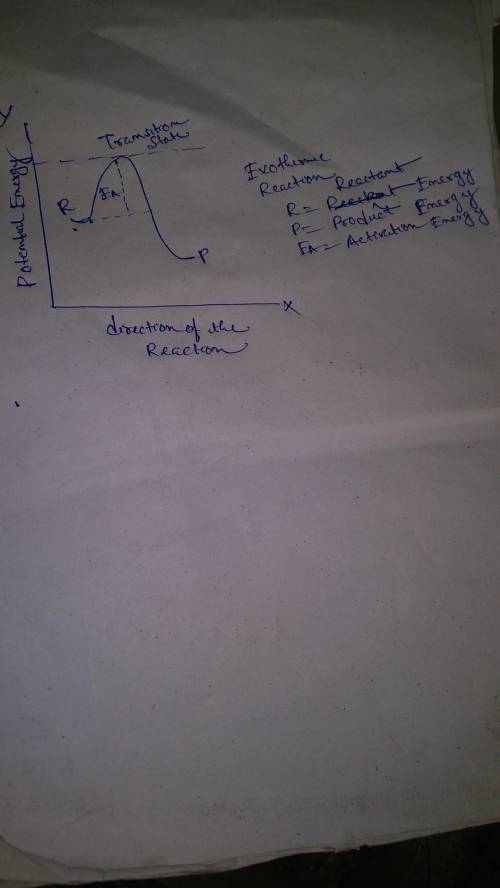
Which statement is true?
in an endothermic reaction, the energy of the products is the same as the energy of the reactants.
in an endothermic reaction, the energy of the products is less than the energy of the reactants.
in an exothermic reaction, the energy of the products is less than the energy of the reactants.
in an exothermic reaction, the energy of the products is the same as the energy of the reactants.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2


Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Which statement is true?
in an endothermic reaction, the energy of the products is the...
in an endothermic reaction, the energy of the products is the...
Questions



Social Studies, 08.10.2019 18:10







English, 08.10.2019 18:10







History, 08.10.2019 18:10