
Chemistry, 18.11.2019 20:31 Thunderalesis7855
Astudent uses visible spectrophotometry to determine the concentration of cocl2(aq) in a sample solution. first the student prepares a set of cocl2(aq) solutions of known concentration. then the student uses a spectrophotometer to determine the absorbance of each of the standard solutions at a wavelength of 510nm and constructs a standard curve. finally, the student determines the absorbance of the sample of unknown concentration. a wavelength of 510nm corresponds to an approximate frequency of 6×1014s−1. what is the approximate energy of one photon of this light? 9×1047j.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Astudent uses visible spectrophotometry to determine the concentration of cocl2(aq) in a sample solu...
Questions




History, 04.05.2020 22:49

Mathematics, 04.05.2020 22:49


Mathematics, 04.05.2020 22:49

History, 04.05.2020 22:49

Chemistry, 04.05.2020 22:49





Mathematics, 04.05.2020 22:49

Mathematics, 04.05.2020 22:49




Mathematics, 04.05.2020 22:49

Mathematics, 04.05.2020 22:49

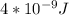
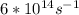
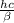 ---- ( 1 )
---- ( 1 )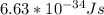
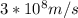
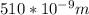
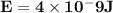
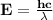
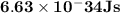
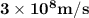
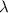 - wavelength = 510nm
- wavelength = 510nm

