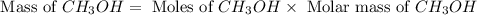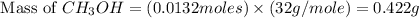Chemistry, 18.11.2019 20:31 PlaneGamer5678
Carbon monoxide gas reacts with hydrogen gas to form methanol. co(g)+2h2(g)? ch3oh(g)a 1.30l reaction vessel, initially at 305 k, contains carbon monoxide gas at a partial pressure of 232 mmhg and hydrogen gas at a partial pressure of 387mmhg .identify the limiting reactant and determine the theoretical yield of methanol in grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3


Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

You know the right answer?
Carbon monoxide gas reacts with hydrogen gas to form methanol. co(g)+2h2(g)? ch3oh(g)a 1.30l reactio...
Questions




Mathematics, 07.06.2021 17:00

History, 07.06.2021 17:00


Mathematics, 07.06.2021 17:00

Mathematics, 07.06.2021 17:00

Mathematics, 07.06.2021 17:00








Mathematics, 07.06.2021 17:00



Mathematics, 07.06.2021 17:00

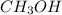 is 0.422 grams.
is 0.422 grams.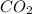 and
and 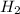 gas.
gas.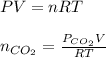
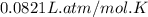
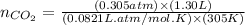
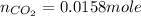
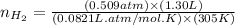
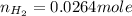
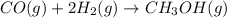
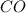
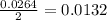 moles of
moles of