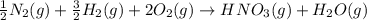Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 3h2(g) 2nh3(g) ah=-92. kj in the second step, ammonia and oxygen react to form nitric acid and water: nh3(9) + 2o2(g) → hno3(9) + h2o(g) ah=-330. kj calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest kj. пkj 1 x ś ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

You know the right answer?
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen...
Questions


Business, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00


Social Studies, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00


Physics, 21.02.2021 14:00

Physics, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00


Mathematics, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00


Mathematics, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00


World Languages, 21.02.2021 14:00


Mathematics, 21.02.2021 14:00

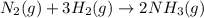 ,
, 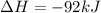
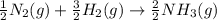 ,
, 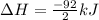
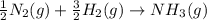 ,
, 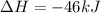 ............ (1)
............ (1)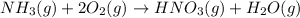
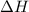 = -330 kJ ............ (2)
= -330 kJ ............ (2)