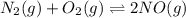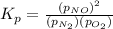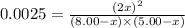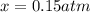Chemistry, 18.11.2019 20:31 sierravick123owr441
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures. n2(g) + o2(g) 2no(g)the equilibrium constant kp for the reaction is 0.0025 at 2127�c. if a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of nitrogen? a) 0.16 atm b) 0.31 atm c) 3.1 atm d) 7.7 atm e) 7.8 atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2


Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatu...
Questions

English, 30.06.2019 23:30




Biology, 30.06.2019 23:30


Mathematics, 30.06.2019 23:30






Mathematics, 30.06.2019 23:30







Mathematics, 30.06.2019 23:30

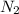 = 8.00 atm
= 8.00 atm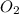 = 5.00 atm
= 5.00 atm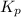 = 0.0025
= 0.0025