
Chemistry, 18.11.2019 20:31 noahdeem135
Industrial production of nitric acid, which is used in many products including fertilizers and explosives, approaches 10 billion kg per year worldwide. the first step in its production is the exothermic oxidation of ammonia, represented by the following equation. 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(g) δh⁰rxn = −902.0 kj if this reaction is carried out using 7.056 ✕ 103 g nh3 as the limiting reactant, what is the change in enthalpy?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Industrial production of nitric acid, which is used in many products including fertilizers and explo...
Questions

History, 29.01.2021 01:10


Computers and Technology, 29.01.2021 01:10



Social Studies, 29.01.2021 01:10

Mathematics, 29.01.2021 01:10

Mathematics, 29.01.2021 01:10

Social Studies, 29.01.2021 01:10


Mathematics, 29.01.2021 01:10

Mathematics, 29.01.2021 01:10

Mathematics, 29.01.2021 01:10


Computers and Technology, 29.01.2021 01:10





Mathematics, 29.01.2021 01:10

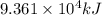
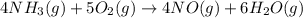
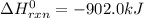
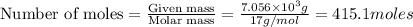
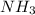 produces = 902.0 kJ of energy
produces = 902.0 kJ of energy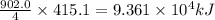 of energy
of energy

