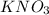Which of the following gives the balanced equation for this reaction?
k3po4 + ca(no3)2 → ca3...

Which of the following gives the balanced equation for this reaction?
k3po4 + ca(no3)2 → ca3(po4)2 + kno3
a) kpo4 + cano3 + kno3
b) k3po4 + ca(no3)2 → ca3(po4)2 + 3kno3
c) 2k3po4 + ca(no3)2 → ca3(po4)2 + 6kno3
d) 2k3po4 + 3ca(no3)2 → ca3(po4)2 + 6kno3
e) k3po4 + ca(no3)2 → ca3(po4)2 + kno3

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which of the following is considered a benefit to using wind energy as a source of power
Answers: 1

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1
You know the right answer?
Questions




Computers and Technology, 13.11.2019 21:31



English, 13.11.2019 21:31






Mathematics, 13.11.2019 21:31


Spanish, 13.11.2019 21:31





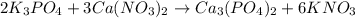
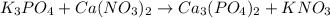
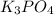 , coefficient 3 is put before the
, coefficient 3 is put before the 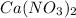 and 6 is put before the
and 6 is put before the