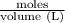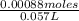A25.0 ml solution of .0600 m edta was added to a 57.0 ml sample containing an unknown concentration of v3+. all v3+ present formed a complex, leaving excess edta in solution.
this solution was back-titrated with a 0440 m ga3+ solution until all the edta reacted requiring 14.0 ml of the ga3+ solution.
what was the original concentration of the v3+ solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
A25.0 ml solution of .0600 m edta was added to a 57.0 ml sample containing an unknown concentration...
Questions

Health, 06.09.2019 17:30



















 as follows.
as follows. will be as follows.
will be as follows.