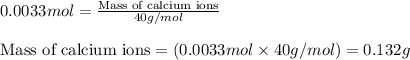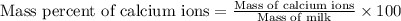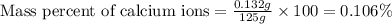Chemistry, 18.11.2019 22:31 kgonzalez200061
The amount of calcium present in milk can be determined through gravimetric analysis by adding oxalate to a sample and measuring the mass of calcium oxalate precipitated. what is the mass percent of calcium in milk if 0.429 g of calcium oxalate, cac2o4, forms in a 125-g sample of milk when excess aqueous sodium oxalate is added? na2c2o4 (aq) + ca2+ (aq) → cac2o4 (s) + 2 na+ (aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3


You know the right answer?
The amount of calcium present in milk can be determined through gravimetric analysis by adding oxala...
Questions


Mathematics, 19.12.2020 22:00






Mathematics, 19.12.2020 22:00



Mathematics, 19.12.2020 22:00


Mathematics, 19.12.2020 22:00




Mathematics, 19.12.2020 22:00

Mathematics, 19.12.2020 22:00


Law, 19.12.2020 22:00

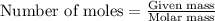 .....(1)
.....(1)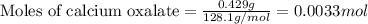
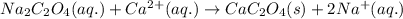
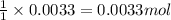 of calcium ions
of calcium ions