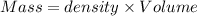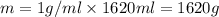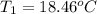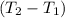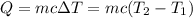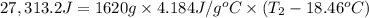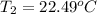Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a constant-pressure calorimeter of negligible heat capacity. the initial temperature of both solutions is the same at 18.46°c. the heat of neutralization when 1.00 mol of hno3 reacts with 0.500 mol ba(oh)2 is −56.2 kj/mol. assume that the densities and specific heats of the solution are the same as for water (1.00 g/ml and 4.184 j/g · °c, respectively). what is the final temperature of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a const...
Questions

Social Studies, 28.04.2022 14:00

Mathematics, 28.04.2022 14:00

World Languages, 28.04.2022 14:00


Health, 28.04.2022 14:00




Physics, 28.04.2022 14:50


Biology, 28.04.2022 15:00

History, 28.04.2022 15:10


Mathematics, 28.04.2022 15:20

Social Studies, 28.04.2022 15:20





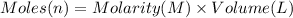
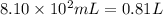
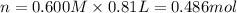
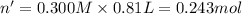
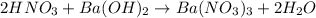
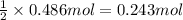 barium hydroxide.
barium hydroxide.