Chemistry, 18.11.2019 23:31 senituliii
Aconcentration cell is one in which both the anode and cathode are the same but with different concentrations. calculate the cell potential with [zn2+] = 0.10 m for the cathode and the [zn2+] = 0.010 m for the anode?
a. + 0.06 v
b. - 0.03 v
c. + 0.03 v
d. 0.0 v

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1


Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Aconcentration cell is one in which both the anode and cathode are the same but with different conce...
Questions

Mathematics, 17.11.2020 18:20

Arts, 17.11.2020 18:20

English, 17.11.2020 18:20

Mathematics, 17.11.2020 18:20


Mathematics, 17.11.2020 18:20

Mathematics, 17.11.2020 18:20

Chemistry, 17.11.2020 18:20








Mathematics, 17.11.2020 18:20

Mathematics, 17.11.2020 18:20


Mathematics, 17.11.2020 18:20

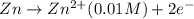


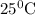 :
:![E_{cell}=\frac{-0.059}{n}log{\frac{[Zn^{2+}]_{0.01M}}{[Zn^{2+}]_{0.10M}}}](/tpl/images/0380/1872/f8c0a.png)