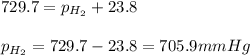Small amount wet of hydrogen gas (h2) can be prepared by the reaction of zinc with excess hydrochloric acid and trapping the gas produced in an inverted tube initially filled with water. if the total pressure of the gas in the collection tube is 729.7 mmhg at 25°c, what is the partial pressure of the hydrogen? the vapor pressure of water is 23.8 mmhg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Small amount wet of hydrogen gas (h2) can be prepared by the reaction of zinc with excess hydrochlor...
Questions


Mathematics, 16.12.2020 18:30



Mathematics, 16.12.2020 18:30

Mathematics, 16.12.2020 18:30

Computers and Technology, 16.12.2020 18:30

Mathematics, 16.12.2020 18:30







Social Studies, 16.12.2020 18:30




Mathematics, 16.12.2020 18:30

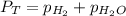
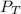 = 729.8 mmHg
= 729.8 mmHg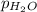 = 23.8 mmHg
= 23.8 mmHg