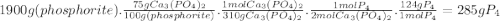Phosporus can be prepared from calcium phosphate by the following reaction: 2ca3(po4)2+6sio2+10c → 6casio3+p4+10co phosphorite is a mineral that contains ca3(po4)2 by mass? assume an excess of the other reactants plus other non-phosphorus-containing compounds. what is the maximum amount of p4 that can be produced from 1.9kg of phosphorite sample is 75% ca3(po4)2 by mass? assume an excess of the other reactants

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
Phosporus can be prepared from calcium phosphate by the following reaction: 2ca3(po4)2+6sio2+10c →...
Questions

Mathematics, 10.07.2019 14:30


Mathematics, 10.07.2019 14:30

Mathematics, 10.07.2019 14:30

Mathematics, 10.07.2019 14:30


Computers and Technology, 10.07.2019 14:30


History, 10.07.2019 14:30

Mathematics, 10.07.2019 14:30

Mathematics, 10.07.2019 14:30


Mathematics, 10.07.2019 14:30


History, 10.07.2019 14:30


Mathematics, 10.07.2019 14:30

Mathematics, 10.07.2019 14:30

Health, 10.07.2019 14:30

Mathematics, 10.07.2019 14:30