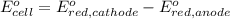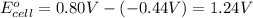Chemistry, 19.11.2019 01:31 burnsmykala23
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given that the standard reduction potential of ag+ to ag (s) is +0.80 v and the standard reduction potential of fe2+ to fe (s) is −0.44 v, calculate the standard cell potential, e°cell.−1.24 v1.24 v2.04 v0.36 v

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2


Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given...
Questions

Biology, 06.09.2019 09:10

Mathematics, 06.09.2019 09:10


Chemistry, 06.09.2019 09:10

Mathematics, 06.09.2019 09:10

English, 06.09.2019 09:10

Chemistry, 06.09.2019 09:10

Chemistry, 06.09.2019 09:10



Chemistry, 06.09.2019 09:10

Mathematics, 06.09.2019 09:10


English, 06.09.2019 09:10


English, 06.09.2019 09:10

Social Studies, 06.09.2019 09:10



Social Studies, 06.09.2019 09:10

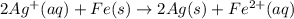
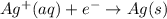
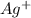 to Ag=
to Ag=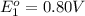
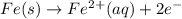
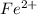 to Fe=
to Fe=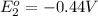
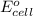 of the reaction, we use the equation:
of the reaction, we use the equation: