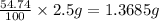Chemistry, 19.11.2019 01:31 sixtomomtermont
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the mixture weighed 72.248g. when empty the beaker weighed 69.748g. the mixture was determined to contain 45.26% sand.
(a) what is the % and mass of the hydrate in the mixture?
(b) if the mixture was selectively decomposed by heating, how many grams and moles of water would be lost?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3

Chemistry, 23.06.2019 16:30
If the enthalpy value for a reaction is negative, what does that indicate about the reaction?
Answers: 1

Chemistry, 23.06.2019 17:30
How do you determine the degree of a power function from a table? when do you know you have successfully determined the degree of the power function?
Answers: 1

Chemistry, 23.06.2019 19:00
1. give an example of a possible hypothesis involving food. 2. what is a simple experiment that you could conduct to test your hypothesis?
Answers: 1
You know the right answer?
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the m...
Questions

Business, 16.12.2020 18:30

Mathematics, 16.12.2020 18:30

Mathematics, 16.12.2020 18:30

Physics, 16.12.2020 18:30


Health, 16.12.2020 18:30

Physics, 16.12.2020 18:30

English, 16.12.2020 18:30

Spanish, 16.12.2020 18:30


Mathematics, 16.12.2020 18:30


Mathematics, 16.12.2020 18:30

English, 16.12.2020 18:30

English, 16.12.2020 18:30



Mathematics, 16.12.2020 18:30

History, 16.12.2020 18:30