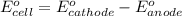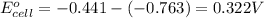Chemistry, 19.11.2019 01:31 EMscary4996
Given the e0 values of the following two half-reactions: zn zn2+ + 2e- e0 = 0.763 volt fe fe2+ + 2e- e0 = 0.441 volt a) write a balanced complete oxidation-reduction reaction? b) explain whether the corrosion of an iron pipe (i. e., fe fe2+) in the presence of zn/zn2+ is possible or not (thermodynamically)? c) explain whether or not zn will protect the corrosion of iron pipe if metallic zn is in contact with the iron pipe?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1


Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Given the e0 values of the following two half-reactions: zn zn2+ + 2e- e0 = 0.763 volt fe fe2+...
Questions



Chemistry, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50


Biology, 17.12.2020 22:50

Biology, 17.12.2020 22:50


History, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50


Mathematics, 17.12.2020 22:50



Mathematics, 17.12.2020 22:50

Health, 17.12.2020 22:50



Health, 17.12.2020 22:50

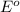 potential will always get reduced and will undergo reduction reaction. Here, zinc will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, zinc will undergo reduction reaction will get reduced.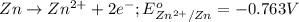
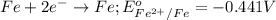
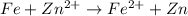
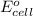 of the reaction, we use the equation:
of the reaction, we use the equation: