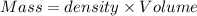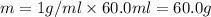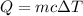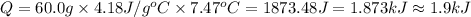Chemistry, 19.11.2019 02:31 Miloflippin9766
A50.0 ml of 0.88 m h2o2 and 10.0 ml of 0.50 m fe(no3)3 were combined and a temperature change of 7.47 was observed. the specific heat of water is 4.18 j/(g * ∘c) calculate the heat of reaction (in kilojoules). record your answer with the proper significant figures and include the correct sign if needed. assume the density and specific heat of the solution are the same as that of water. kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
A50.0 ml of 0.88 m h2o2 and 10.0 ml of 0.50 m fe(no3)3 were combined and a temperature change of 7.4...
Questions

History, 22.10.2020 05:01


Mathematics, 22.10.2020 05:01

Mathematics, 22.10.2020 05:01

English, 22.10.2020 05:01

Biology, 22.10.2020 05:01

Mathematics, 22.10.2020 05:01

Mathematics, 22.10.2020 05:01

Computers and Technology, 22.10.2020 05:01

Advanced Placement (AP), 22.10.2020 05:01

Mathematics, 22.10.2020 05:01

Mathematics, 22.10.2020 05:01

Mathematics, 22.10.2020 05:01

History, 22.10.2020 05:01


Computers and Technology, 22.10.2020 05:01

Mathematics, 22.10.2020 05:01

English, 22.10.2020 05:01

Mathematics, 22.10.2020 05:01

History, 22.10.2020 05:01