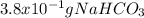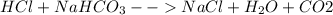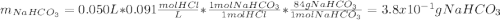Chemistry, 19.11.2019 02:31 heathkid23
Sodium hydrogen carbonate , also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid , which the stomach secretes to digest food. drinking a glass of water containing dissolved neutralizes excess through this reaction: (aq) (aq) (aq) (l) (g) the gas produced is what makes you burp after drinking the solution. suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be of a m solution. what mass of would she need to ingest to neutralize this much ? be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Sodium hydrogen carbonate , also known as sodium bicarbonate or "baking soda", can be used to reliev...
Questions

Biology, 20.07.2019 05:40

Social Studies, 20.07.2019 05:40

Mathematics, 20.07.2019 05:40




Biology, 20.07.2019 05:40


Biology, 20.07.2019 05:40


Biology, 20.07.2019 05:40

Biology, 20.07.2019 05:40

Biology, 20.07.2019 05:40

Social Studies, 20.07.2019 05:40

Social Studies, 20.07.2019 05:40

Biology, 20.07.2019 05:40



Social Studies, 20.07.2019 05:40

Computers and Technology, 20.07.2019 05:40