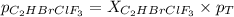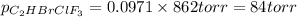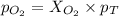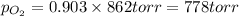Chemistry, 19.11.2019 02:31 marlenemedina247
Amixture of 15.0 g of the anesthetic halothane (c2hbrclf3 197.4 g/mol) and 22.6 g of oxygen gas has a total pressure of 862 torr. what are the partial pressures of each gas? a. phalothane = 778 torr, po2 = 84 torr b. phalothane = 162 torr, po2 = 700 torr c. phalothane = 84 torr, po2 = 778 torr d. phalothane = 155 torr, po2 = 707 torr e. phalothane = 707 torr, po2 = 155 torr.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Amixture of 15.0 g of the anesthetic halothane (c2hbrclf3 197.4 g/mol) and 22.6 g of oxygen gas has...
Questions

History, 15.04.2021 18:40


Mathematics, 15.04.2021 18:40





World Languages, 15.04.2021 18:50







Arts, 15.04.2021 18:50




Mathematics, 15.04.2021 18:50

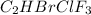 and
and 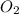 are, 84 torr and 778 torr respectively.
are, 84 torr and 778 torr respectively.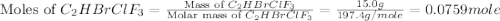
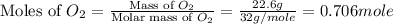
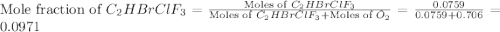
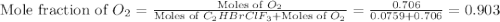
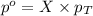
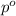 = partial pressure of gas
= partial pressure of gas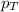 = total pressure of gas
= total pressure of gas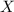 = mole fraction of gas
= mole fraction of gas