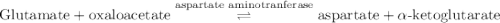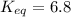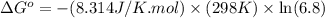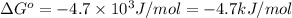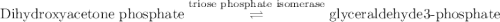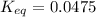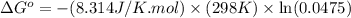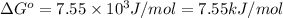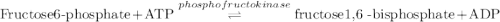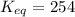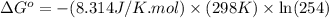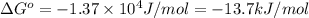Chemistry, 19.11.2019 03:31 joseenrique02
Calculation of δg′° from an equilibrium constant calculate the standard free-energy change for each of the following metabolically important enzyme-catalyzed reactions, using the equilibrium constants given for the reactions at 25 °c and ph 7.0. ( a ) glutamate + oxaloacetate aspartate aminotranferase ⇌ aspartate + α -ketoglutarate k ′ eq = 6.8 ( b ) dihydroxyacetone phosphate triose phosphate isomerase ⇌ glyceraldehyde 3 -phosphate k ′ eq = 0.0475 ( c ) fructose 6 -phosphate + atp phosphofructokinase ⇌ fructose 1 , 6 -bisphosphate + adp k ′ eq = 254

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
Calculation of δg′° from an equilibrium constant calculate the standard free-energy change for each...
Questions

Physics, 03.02.2022 19:00


Biology, 03.02.2022 19:00

English, 03.02.2022 19:00


Mathematics, 03.02.2022 19:00



Mathematics, 03.02.2022 19:00



Mathematics, 03.02.2022 19:10

Chemistry, 03.02.2022 19:10

Mathematics, 03.02.2022 19:10

Mathematics, 03.02.2022 19:10

Mathematics, 03.02.2022 19:10

Mathematics, 03.02.2022 19:10



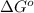 for the reaction is -4.7 kJ/mol
for the reaction is -4.7 kJ/mol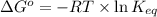
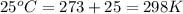
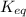 = equilibrium constant
= equilibrium constant