
Chemistry, 19.11.2019 03:31 kordejah348
One of the emission spectral lines for be31 has a wavelength of 253.4 nm for an electronic transition that begins in the state with n 5 5. what is the principal quantum number of the lower-energy state corresponding to this emission? (hint: the bohr model can be applied to one-electron ions. don’t forget the z factor: z 5 nuclear charge 5 atomic number.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
One of the emission spectral lines for be31 has a wavelength of 253.4 nm for an electronic transitio...
Questions

Geography, 28.10.2020 01:50






Mathematics, 28.10.2020 01:50

History, 28.10.2020 01:50



Chemistry, 28.10.2020 01:50


Mathematics, 28.10.2020 01:50

Mathematics, 28.10.2020 01:50




History, 28.10.2020 01:50


Social Studies, 28.10.2020 01:50

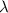 = 253.4 nm =
= 253.4 nm =  (as 1 nm =
(as 1 nm =  )
)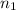 = 5,
= 5, 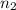 = ?
= ?
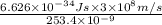
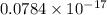 J
J







