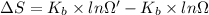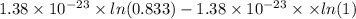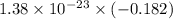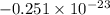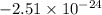Chemistry, 19.11.2019 03:31 wendii87wh
Agaseous system undergoes a change in temperature and volume. what is the entropy change for a particle in this system if the final number of microstates is 0.833 times that of the initial number of microstates? express your answer numerically in joules per kelvin per particle.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1

Chemistry, 23.06.2019 11:40
An electron moved from a lower energy level to a higher energy level. what most likely happened during the transition? a random amount of light was released. a fixed amount of energy was absorbed. a fixed amount of energy was released. a random amount of light was absorbed.
Answers: 1
You know the right answer?
Agaseous system undergoes a change in temperature and volume. what is the entropy change for a parti...
Questions


Mathematics, 02.12.2020 19:50



Mathematics, 02.12.2020 19:50


History, 02.12.2020 19:50

Mathematics, 02.12.2020 19:50

Social Studies, 02.12.2020 19:50



Business, 02.12.2020 19:50

Mathematics, 02.12.2020 19:50




Social Studies, 02.12.2020 19:50


Mathematics, 02.12.2020 19:50

Mathematics, 02.12.2020 19:50

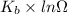
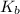 = Boltzmann constant
= Boltzmann constant = number of microstates
= number of microstates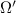 = 0.833
= 0.833